orange book pharmacy definition
Office of Generic Drugs Policy Center for Drug Evaluation Research US. G o v e r n a n c e and L e a d e r s I n te g ra o n h i p C o l a b or ti o n Information Insight Insight Information Communication.

List Of Branches Of Science And Definition Classification Of Science Branches Of Science Biology Facts Medical Knowledge
Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use.
. The FDAs Orange Book identifies approved drug products. Food and Drug Administration 10001 New Hampshire Ave Hillandale Bldg 4th Floor Silver Spring MD 20993 -0002 Phone. Type The group or category of approved drugs.
1 approved prescription drug products with therapeutic equivalence evaluations. Objectives What is a Generic Drug. Rules and Guidance for Pharmaceutical Manufacturers and Distributors commonly known as the Orange Guide brings together all the main European and UK directives regulations and legislation relating to the manufacture and distribution of medicines.
Originally this book was published in October 1980 with orange cover and thus the name orange book. FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations. FDA has draft guidance explaining that certain currently marketed drug ingredients were marketed before current FDA legislation.
An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. Orange Book Approved Drug Products with Therapeutic Equivalence Evaluations Brand-generic therapeutic equivalencies Found in accessdatafdagov. The Orange Book is composed of four parts.
CDR Kendra Stewart RPh PharmD. Use of unapproved drugs require complete CMC information depending on the nature of the study. PCS - Dr Z.
Before understanding different drug ratings it is necessary. The cost that the patient pays at the pharmacy point of sale. The Orange book has been revised.
The Office of Inspector General. Sponsors using these products should consult FDA about the need for an IND. Approved Drug Products with Therapeutic Equivalence Evaluations.
Then use the Ingredient Search for. The orange book is a list of generic drugs approved by FDA. The Orange Book Risk Management Principles.
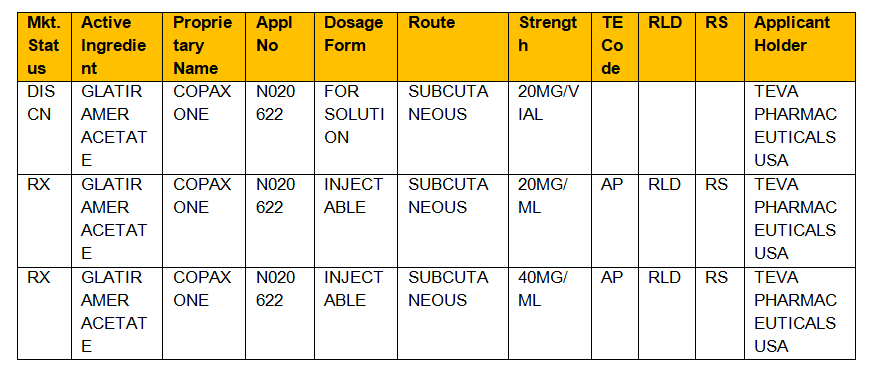
Format is RX OTC DISCN. R i s k r e. Food and Drug Administration.
The Orange Book is a reference source that gives insight on whether or not two drugs have Therapeutic Equivalences. Updated with Orange Book. The FDAs list of Approved Drug Products with Therapeutic Equivalence Evaluations.
2 approved over-the-counter OTC drug products for those drugs that. The Orange Book is a compendium of significant unimplemented nonmonetary recommendations for improving departmental operations. The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange Guide is now available through.
The Orange Book Introduction. The orange book consist of five main sections. The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for.
Typically refers to a drug product that can be purchased without a prescription. Get emails about this page. First if you have the trade name search the Electronic Orange Books Rx or OTC section using the Proprietary Name search.
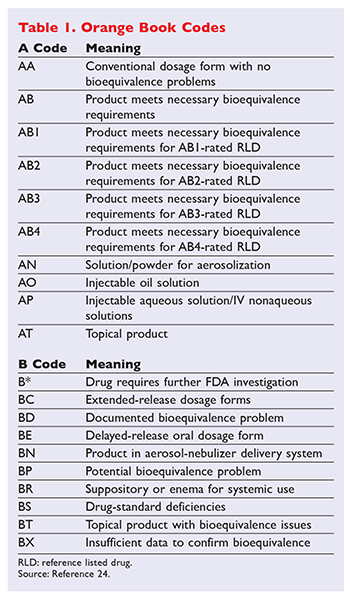
In the electronic Orange Book a reference standard is identified by RS in the RS column. The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the FDA. Basics in drug approval process with reference to the Orange Book Presented by.
Rucha Pathak Roll No. Not much more than 30 pages in length this voluntary guide was an aid to manufacturers to understand the needs of the regulatory authoritys requirements for the manufacture of. New Delhi India Dec 6 ANI.
Indias largest online marketplace for automobiles Droom has announced expansion of the scope of Orange Book Value popularly known as OBV to give the fair market value of used mobile phones to the customers. It is widely accepted as the authoritative source for determining therapeutic equivalence among multisource drug products. First published in 1971 the original Orange Guide contained British Good Manufacturing Practice and was entitled Guide to Good Pharmaceutical Manufacturing Practice.
This determines the ingredient s. Sets with similar terms.

Pin By Suzanne Peirsel On Art Lee Dubin Pharmacy Art Graphic Design Portfolio Inspiration Vintage Posters

The Introduction Of An Orange Book

Vital Signs Physical Observations Nurse Study Notes Vital Signs Nursing Students

Thank You To All Of Our Local Pharmacists With Illness Going Around This Time Of Year We Thank You For Keeping Us Hea Pharmacist Pharmacist Quote Funny Quotes
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt

The Introduction Of An Orange Book

The Apothecary Lee Dubin Metal Sign Etsy Pharmacy Art Apothecary Graphic Design Portfolio Inspiration

What Is Neurogenesis Definition Mechanisms And Its Role In Brain Plasticity Neuroplasticity Biochemical Physical Activities

The Introduction Of An Orange Book

The Introduction Of An Orange Book

Jessica Hische Print Work Creative Illustration Illustration Jessica Hische
Orange Book And Its Applications Legal Advantage

What Is Copywriting Social Media Marketing Help Social Media Management Tools Copywriting

Pharmaceutical Press Rules And Guidance For Pharmaceutical Manufacturers And Distributors 2022 The Mhra Orange Guide
Insights Into Effective Generic Substitution

Anne Neilson S Angels Devotions And Art To Encourage Refresh And Inspire By Anne Neilson Thomas Nelson Hardcover Book Fine Art Thomas Nelson Hardcover Book
/doctor-826e0c116cd549d98e327f1184c622d9.jpg)
